The Genomic Center for Cancer Research & Diagnosis
Our Mission
The Genomic Centre for Cancer Research and Diagnosis (GCCRD) was created as a Regional/National Facility for all cutting edge imaging applications and was initiated by Dr. Sabine Mai. It is part of the Research Institute at CancerCare Manitoba and the University of Manitoba, in Winnipeg, Manitoba. It began with research into genomic instability and mechanisms of neoplasia but has since expanded to meet various peoples with differing project needs.
Activities of the GCCRD now include projects in molecular biology, cell biology, histopathology, and genetic diseases. The goals of the GCCRD are basic and translational research, as well as the education of students and highly qualified personnel in genomic instability, cancer genetics, and imaging. The facility was established through funds from the Canada Foundation for Innovation in 1999 amounting to $2.3 million. Updates and new equipment are continually being added. It has since been fully supported by CancerCare Manitoba and for further information on funding see below.
The GCCRD is equipped with state-of-the-art equipment and the technical capabilities of the GCCRD are diverse but complementary, and they include:
- Microscopy and imaging of live cells
- Study of relative nuclear and cytosolic protein levels by quantitative
fluorescent immuno-staining - Spectral karyotyping (SKY)
- Spectral imaging
- Comparative genomic hybridization (CGH)
- Fluorescent in situ hybridization (FISH):
- multicolour-FISH (M-FISH)
- multicolour-Banding (m-Banding)
- quantitative-FISH (Q-FISH)
- Automatic slide scanning
- Histology/Pathology
- 3-Dimensional (3-D) imaging
- Deconvolution
- Confocal microscopy (LSM710)
- Superresolution Microscopy (ELYRA PS.1)
- Structural Illumination Microscopy (SIM)
- Photo Activated Localization Microscopy (PALM)Stochastic Optical Reconstruction Microscopy (STORM)
The GCCRD is also equipped with a teaching microscope for the training of diverse populations of students. This includes high school, summer, project, graduate, medical students and residents, as well as post-doctoral fellows, and the advanced training of scientists. The Strategic Training Program Grant (Innovative Technologies in Multi-disciplinary Health Research Training) sponsored by the Canadian Institutes of Health Research (CIHR) makes it possible to hold a beginner workshop in the fall and an intermediate/advanced workshop the following spring; these workshops count as credits merited by the University of Manitoba. Please see our training program website for more details. GCCRD facility was funded four times through the Canada Foundation for Innovation (CFI) to a total of $16.8M. In addition to its other multifaceted capabilities, the GCCRD became the 3D Imaging Node for Canada in 2004. Scientific partners include:
- The University of Manitoba
- CancerCare Manitoba
- CancerCare Manitoba Foundation
- Bioscience Association Manitoba
- British Columbia Cancer Agency
- Queens University, Kingston, Ontario, Canada
- University of Toronto, The Hospital for Sick Children
- McGill University, Jewish General Hospital
- Tartu University Hospital, Tartu, Estonia
- Microbiology and Tumour Biology Center, Karolinska Institute (Sweden)
- Department of Clinical Genetics, Institute of Biomedicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden;
- Systems Biology Research Centre, School of Life Sciences, University of Skövde, Skövde, Sweden
- Institute of Molecular Biology GmbH (IMB), Mainz, Germany
- Prince of Songkla University Hospital, Hat Yai, Songkla, Thailand
- Chiang Mai University (Thailand)
- National Institutes of Health and National Cancer Institute (USA)
Corporate partners include:
- VWR Scientific Products,
- Sarstedt Inc.,
- Cedarlane Laboratories Inc.,
- Kindler GmbH & Co.,
- Invitrogen Canada, Inc.,
- Applied Spectral Imaging Inc.,
- Empix Imaging Inc.,
- MetaSystems Group Inc.,
- Carl Zeiss Canada Ltd.,
- Photonic Instruments Inc.
- 3D Signatures Inc.
The Genomic Centre's educational component is comprised of endeavours with:
- Manitoba
- John’s Ravenscourt, Winnipeg, MB.
- Mary’s Academy, Winnipeg, MB.
- Grant Park High School, Winnipeg, MB.
- Europe
- Städtischen Gymnasium, Herzogenrath, Germany
- Manchester High School for Girls, Manchester, UK
The GCCRD is a member of the Bioscience Association Manitoba and the Canadian Network of Scientific Platforms
Universities:
- Canada
- Université de Sherbrook, Sherbrook, PQ
- University of British Columbia, Vancouver, BC
- University of Winnipeg, MB
- Europe
- Universität zu Köln, Cologne, Germany
- Università di Cagliari, Monserrato, Italia
- Università Degli Studi di Modena E Reggio Emilia, Moena, Italia
Equipment
The GCCRD has been designed as a high-technology facility for digital imaging and analysis. The activities havebeen divided into two major areas, basic research and technical services, that are envisioned to promote a better interaction between different fields of research.
The basic research component of the GCCRD is developed on collaboration with scientific groups from Canada, USA, Asia and Europe. Technical services, on the other hand, are offered on a fee basis, and these are mainly forcused on fluorescent in situ hybridization, immunohistochemistry, and microdissection of biological material. The Centre does not carry out clinical diagnostics, but collborations with clinical genetics laboratories are strongly encouraged.
Descriptions of Workstations
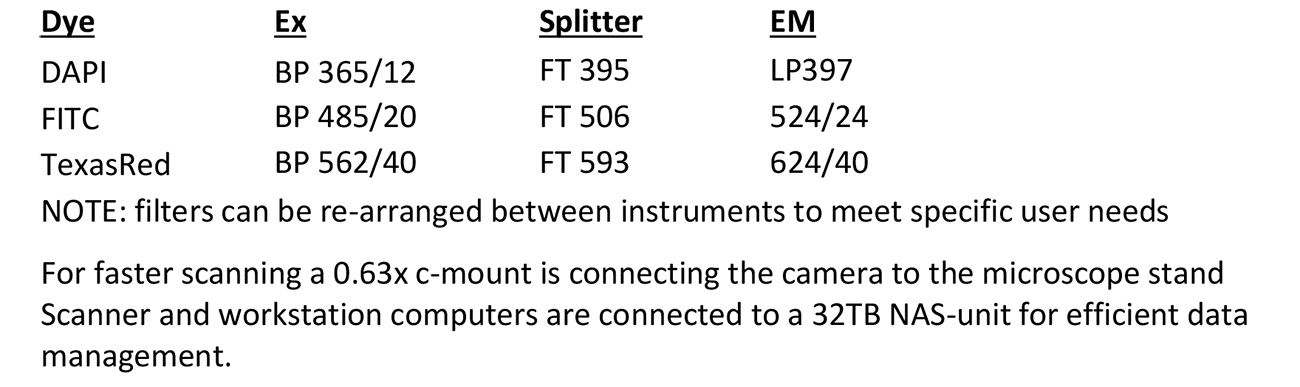
Automated Platform: Applied Spectral Imaging (ASI)
Microscope, camera & light source:
Zeiss AxioImager.Z2 equipped with
- 9-slide Märzhäuser stage
- Motorized x/y and z-focus drive
- 10-filter position filter turret
- ASI ScanView camera
- x-Cite 120Q light source
Software:
GenASIs 7.2.5
Modules: SpotScan

Objectives:
- 4x 0.40 Dry Achroplan
- 10x 0.25 Dry Plan Apochromate
- 20x 0.8 Dry Plan Apochromate
- 40x 0.95 Korr Plan Apochromate
- 63x 1.4 oil Plan Apochromate

Deconvolution Station 1
Microscope, camera & light source:
ZEISS AxioImager.Z2 equipped with
- Motorized z-focus drive
- 10-filter position filter turret
- Zeiss AxioCam 702 mono
- x-Cite 120 light source
Software:
ZEN Blue 2.5
Objectives:
- 20x 0.50 Dry Plan-Neofluar
- 40x 0.75 Dry Plan-Neofluar
- 40x 0.3 oil Plan-Neofluar
- 63x 1.4 oil Plan Apochromate

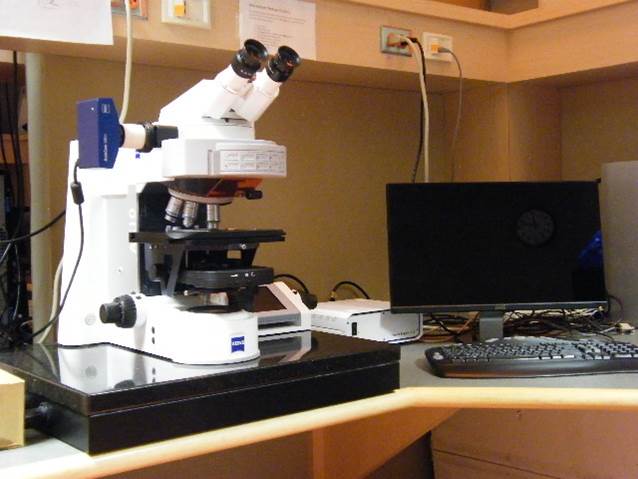

Applications:
2D and 3D fluorescence microscopy,
FISH, Q-FISH, immunofluorescence, Immuno-FISH,
Devconvolution Station 2
Microscope, camera & light source:
ZEISS AxioImager.Z2 equipped with
- Motorized z-focus drive
- 10-filter position filter turret
- ZEISS AxioCam MRm
- HXP120C light source
Software:
AxioVision Release 4.8.2
Objectives:
- 20x 0.50 Dry Plan-Neofluar
- 40x 0.75 Dry Plan-Neofluar
- 63x 1.4 oil Plan Apochromate



Applications:
2D and 3D fluorescence microscopy, FISH, Q-FISH, immunofluorescence, Immuno-FISH.
Deconvolution Stations 3
Microscope, camera & light source:
ZEISS AxioImager.Z1 equipped with
- Motorized z-focus drive
- 10-filter position filter turret
- ZEISS AxioCam HRm
- x-Cite 120 light source
Software:
AxioVision Release 4.8.1
Objectives:
- 10x 0.30 Dry Plan Neofluar
- 20x 0.50 Dry Plan-Neofluar
- 40x 0.75 Dry Plan-Neofluar
- 40x 0.3 oil Plan-Neofluar
- 63x 1.4 oil Plan Apochromate
- 100x 1.4 oil Plan Apochromat



Applications:
2D and 3D fluorescence microscopy,
FISH, Q-FISH, immunofluorescence, Immuno-FISH, optical sectioning using structured illumination
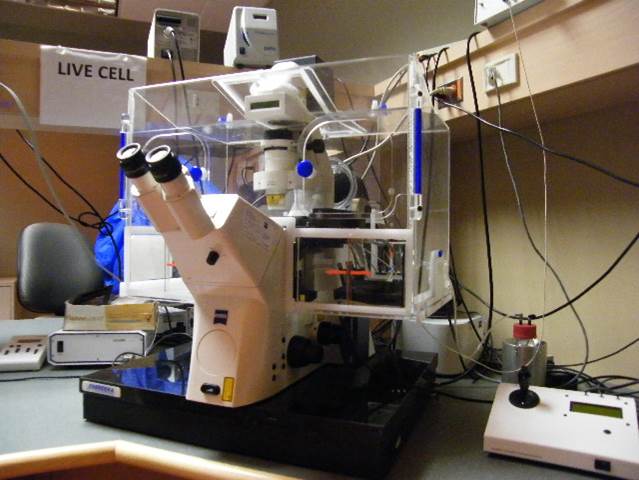
Live Cell Station
Microscope, camera & light source:
Zeiss Axiovert200M equipped with
- xx-filter position filter turret
- Zeiss AxioCam MRm and AxioCam HSm
- x-Cite 120 light source
Software:
AxioVision 4.6.3
Objectives:
- 20x 0.50 Dry Plan-Neofluar
- 40x 0.75 Dry Plan-Neofluar
- 40x 1.30 Dry Plan-Neofluar
- 63x 1.4 oil Plan Apochromate
- 100x 1.3 oil Plan-Neofluar

Metasystems Station
Microscope, camera & light source:
Zeiss Axioplan2 equipped with
- 5-filter position filter turret
- MetaSystems camera
- FluoArc light source
Software:
MetaSystems Isis, Ikaros version 5.4.9
Objectives:
- 20x 0.50 Dry Plan-Neofluar
- 40x 0.75 Dry Plan-Neofluar
- 40x 1.30 Dry Plan-Neofluar
- 63x 1.4 oil Plan Apochromate
- 100x 1.3 oil Plan-Neofluar


MMI CellCut Plus
Microscope, camera & light source:
Olympus IX 71 equipped with:
- mmi CellCamera 1.4
- IX2-RFAC 6-filter position mirror turret
- x-Cite 120Q light source
- mmi CellCut laser
- Average power: <10mW
- Pulse width: <1ms
- Wavelength: 355nm
- Pulse energy: >5uJ
Software:
mmi CellTools 4.2.2
Objectives:
- 4x/0.9 U Plan FLN
- 10x/0.30 U Plan FLN
- 20x/0.5 U Plan FLN
- 40x/0.6 LUC Plan FLN
- 60x/0.9 U Plan FLN



Applications:
Automated cutting of cells for single cell sequencing of fresh frozen, paraffin embedded, archived slides, cytospins, smears, live cells
http://www.molecular-machines.com/single_cell_sorting_products/_mmi_cellcut_-_laser_microdissection/technology_27,
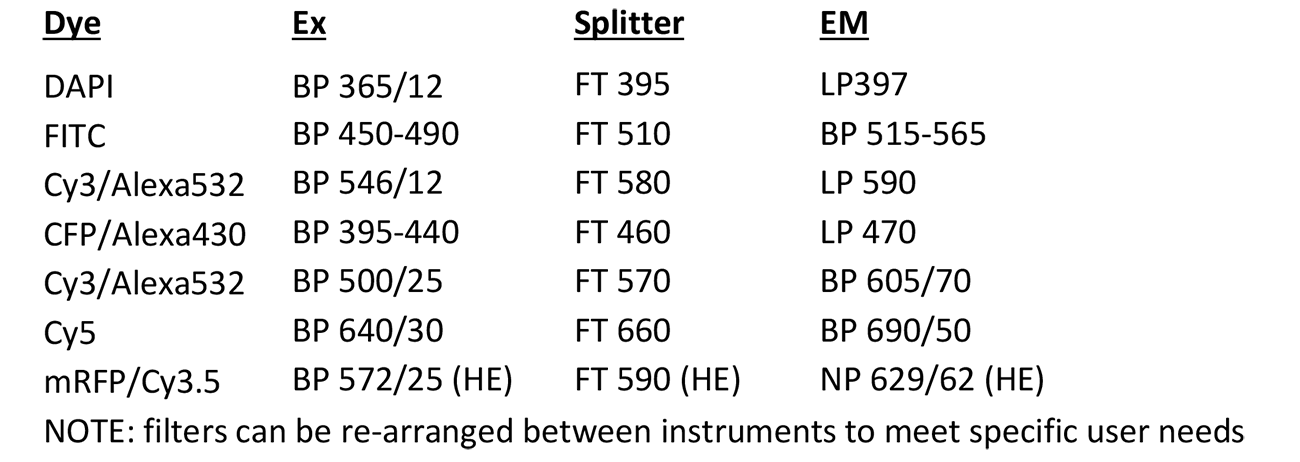
Pathology Station
Microscope, camera & light source:
Zeiss Axioscope2 equipped with
- 4-filter position filter turret
- Zeiss AxioCam ICc3 colour camera
- AttoArc 2 HBO100W light source
Software:
AxioVision Release 4.8
Objectives:
- 10x 0.3 Dry Plan-Neofluar
- 20x 0.50 Dry Plan-Neofluar
- 50x 0.7 Dry EPI Plan
- 63x 1.4 oil Plan Apochromate
- 100x 1.3 oil Plan-Neofluar
Applications: Brightfield microscopy of e.g. H&E or Giemsa stained samples, fluorescence microscopy

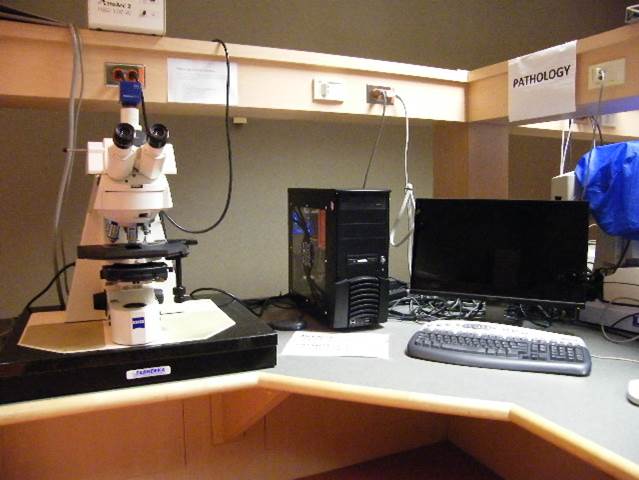

Applications:
Brightfield microscopy of e.g. H&E or Giemsa stained samples, fluorescence microscopy
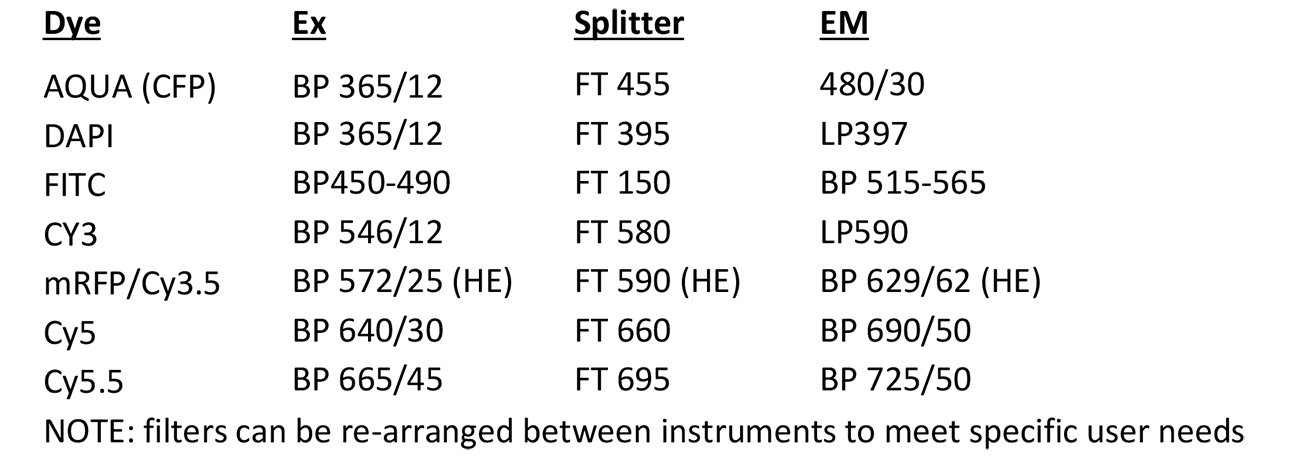
Scanning Metasystems Station
Microscope, camera & light source:
Zeiss Axioplan 2 equipped with
- Motorized 8-slide stage
- Zeiss AxioCam MRm
- x-Cite 120Q light source
Software:
Metafer Slide Scanning System version 3.8 (Release 2010)
Objectives:
- 10x 0.5 Dry Fluar
- 20x 0.50 Dry Plan-Neofluar
- 40x 0.75 Dry Neofluar
- 40x 1.3 oil Plan-Neofluar
- 63x 1.4 oil Plan Apochromate
- 100x 1.3 oil Iris



Applications:
Fluorescence in situ hybridization (FISH), multicolour-FISH (M-FISH), quantitative-FISH (Q-FISH), karyotyping (fluorescent and brightfield); multiple slide platform for metaphase, cells, and FISH signal detection
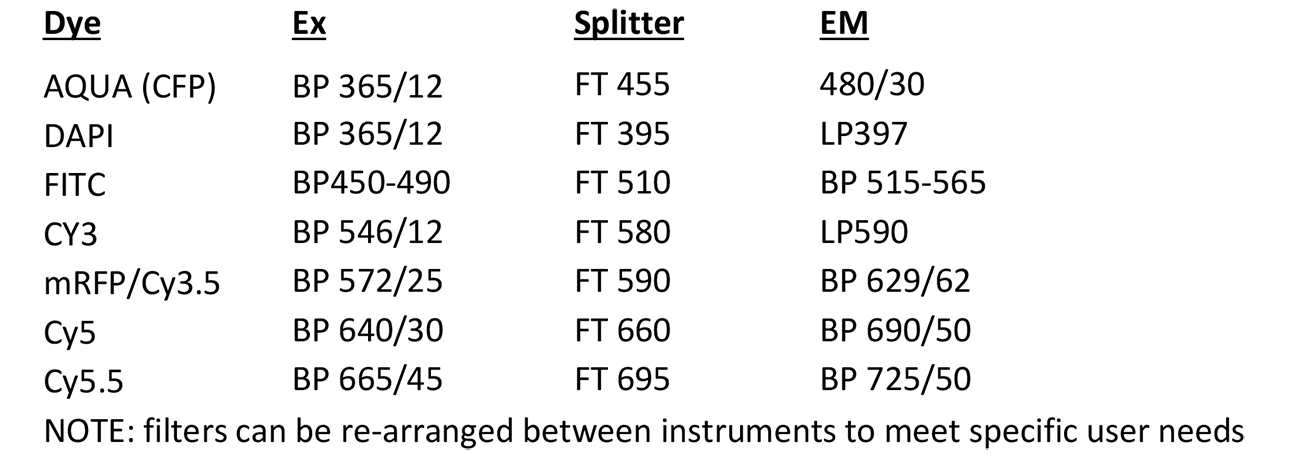
Spectral Karyotyping (SKY)
Microscope, camera & light source:
Zeiss Axioplan 2 equipped with
- ASI SpectraCubeTM Model COOL-1300Q
- 4 filter turret
Software:
- Case Data Manager
- Version 6.0.0.16
- Modules:
- Spectral Imaging,
- SkyView
- FishView
- BandView
Objectives:
- 20x 0.50 Dry Plan-Neofluar
- 40x 0.75 Dry Plan-Neofluar
- 40x 1.3 oil Plan-Neofluar
- 63x 1.4 oil Plan Apochromate
- 100x 1.4 oil Iris

Super Resolution Imaging Station
Microscope, camera & light source:
Zeiss AxioObserver.Z1 equipped with
Software:
Carl Zeiss Zen 2010D (64-bit)
LSM 7 Elyra Release Version 7.0
Objectives:
- 10x 0.25 N-A Chorplan
- 20x 0.8 Plan Apo
- 40x 1.4 oil Plan Apochromate
- 63x 1.4 oil Plan Apochromate
- 100x 1.46 oil Plan Apochromate

Helpful Links
Applications:
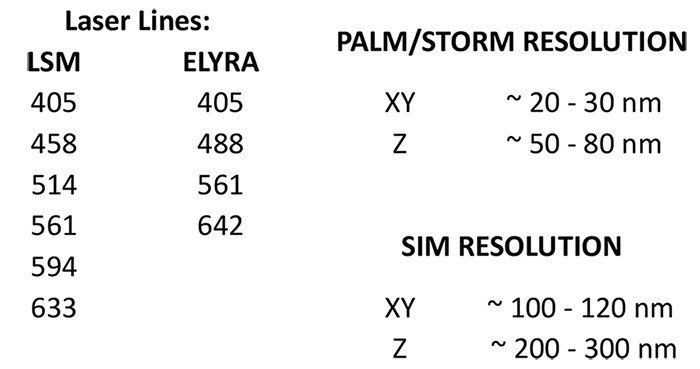
2- and 3D high-resolution (20 nm) fluorescence imaging, laser scanning microscopy, FRET, FRAP, RICS, PALM, SIM
Guidelines for Working in the GCCRD
- Most fluorescent bulbs should warm up and cool down for at least 20 minutes (AttoArc 2 HBO 100W mercury burners). This not only helps preserve the life span of the bulbs, but will also improve imaging. The XCite Illumination burners only need a 90 second warm up and a 5 minutes to cool down.
- Inform the GCCRD personnel right away If the bulb hours are getting high (around 80 hours on the AttoArc or 1400 hours on the X-Cite) or the light is flickering.
- INFORME THE GCCRD PERSONNEL IMMEDIATELY if the instruments/light sources are not working properly!! This is imperative, since more serious issues could do permanent damage to the equipment. We may also have to place a service call or order a new part. If there is no one available, please write the problem in the problem log in the log-in files.
- All the objectives must be cleaned properly once the user is finished. Use only the lens paper and lens cleaner provided at the workstations.
- Bulbs, microscopes, computers and power bars should be turned off after use, unless someone else is there waiting to use that station.
- Microscopes have to be covered with the blue Zeiss cover when you are finished.
- Users are not allowed to move parts from microscope to microscope or remove parts from the microscope.
- You are not allowed to remove or delete files from the computer unless they are yours.
- Users are to be trained on required workstations by GCCRD personnel only. Fellow lab members are not suitable trainers.
- Users can only use the workstations they have been trained on. If it has been more than three months since you used a station you may want to request a brief review of that station.
- All users are required to record name, type of use (imaging/analysis), bulb hours, project, and the name of the supervisor. This includes all analysis stations in the computer room.
- Food and/or drink are not allowed in the GCCRD.
- Lab coats and/or gloves are not to be worn in the GCCRD.
- Files are not to be stored on any hard drive of individual workstations. It is solely the user's responsibility to back up data on external storage media (external HD, USB-stick etc.)
- The use of GCCRD-computers for personal use, specifically e-mail access as well as downloading software of any kind on the GCCRD workstation computers is absolutely prohibited.
All of the above rules and guidelines will be explained in your training session. Users breaking the rules will lose all or certain privileges of the GCCRD, depending on the severity. The GCCRD personnel and the user committee will determine the loss of privileges. Please understand that we have to be strict about access to the GCCRD and workstations, because the equipment is expensive.
Personnel
Director - Dr Sabine Mai, Ph.D.
Dr. Mai is responsible for the general scientific direction and development of research projects within the GCCRD.
Her focus is upon the development of advanced methods for the early detection of cancer and the mechanisms of genomic instability. She also co-ordinates the Health Research Training program.
Dr. Mai can be reached by email at sabine.mai@umanitoba.ca
Co-Director - Dr James Davie, Ph.D.
Dr. Davie is both the director of the Manitoba Institute of Cell Biology and chair of the GCCRD user committee.
The GCCRD user committee is responsible for overseeing the budget and policy decisions of the GCCRD.
Dr. Davie's laboratory studies the nuclear organization and the structure and function of chromatin.
Dr. Davie can be reached by email at jim.davie@umanitoba.ca.
Technician - Landon Wark
Mr. Wark is primarily responsible for performing wet lab protocols for users outside of the Research Institute and for helping new users troubleshoot their protocols. Landon performs image acquisition and analysis for users outside of the Research Institute. He also trains new users and assists with tours and the workshops.
Landon can be reached by email at lando.wark@gmail.com
GCCRD user committee members are Drs. Sabine Mai,James Davie, Spencer Gibson, James Johnston, Kirk McManus, Michael Mowat and Leigh Murphy.
Fees
AHourly Rates for the Training and Usage of the GCCRD Workstations
| System | University of Manitoba | Other Academic Institutions | Industry |
|---|---|---|---|
| Live-cell | $2.50 | $5.00 | $10.00 |
| Epifluorescent Microscopes | $25.00 | $50.00 | $100.00 |
| Automated Epifluorescent Microscopes | $25.00 | 50.00 | $100.00 |
| Spectral Karyotyping | $35.00 | $70.00 | $150.00 |
| MMI Cellcut Plus | $25.00 | $50.00 | $100.00 |
| Teaching on the above instruments | $100.00 | $200.00 | $300.00 |
| ELYRA | $75.00 | $100.00 | $200.00 |
| LSM710 | $75.00 | $100.00 | $200.00 |
| Teaching on the above instruments | $200.00 |
$400.00 |
$600.00 |
| Image Analysis | $20.00 |
$33.00 |
$50.00 |
| Data Analysis | $20.00 |
$33.00 |
$50.00 |
| The prices exclude labour fees - those are $35/hour (tech), $50/hour (highly qualified personnel). All prices are subject to taxes when applicable. |
|||
Upcoming Events
Training Workshops
Training Workshops 2017
Beginner Level Workshop
Intermediate Level Workshop
Workshop Trainees are introduced to highly advanced software and microscopes. Trainees learn specialized principles of microscopy via lectures, bench work and a variety of techniques such as image acquisition, data analysis and archiving in the GCCRD. Trainees enjoy a unique opportunity to study whole genomes, single cells, cellular components and functions through advanced molecular imaging, micro-array analysis and functional genomics. The workshops cover a wide range of disciplines, such as molecular and cytogenetic methodologies, the use of imaging software, and other techniques pertaining to the GCCRD. Target groups for training are graduate level students in the biological sciences, medical students, MD/PhD students, residents, post doctoral, clinical and visiting fellows, clinician-scientists, research associates and technicians. In addition, the training of high school and undergraduate students, as well as the education of teachers and the public, is a firm foundation of the training program. This adds to the general knowledge of health science and novel technologies, and may facilitate later career choices.
Several workshops are organized each year in collaboration with our industrial partners, Applied Spectral Imaging Inc., Invitrogen Life Technologies Inc., Carl Zeiss Canada Ltd., Applied Spectral Imaging, and MetaSystems Group Inc.
Please go to the Strategic Training Program website (Innovative Technologies in Multidisciplinary Health Research Training) for an in-depth look at past workshops. The website also contains information on how to apply, research and training opportunities, ITMHRT mentors and their contact information.
You may also contact the workshop directly at: workshop@umanitoba.ca