Manitoba Tumour Bank
What is the Manitoba Tumour Bank?
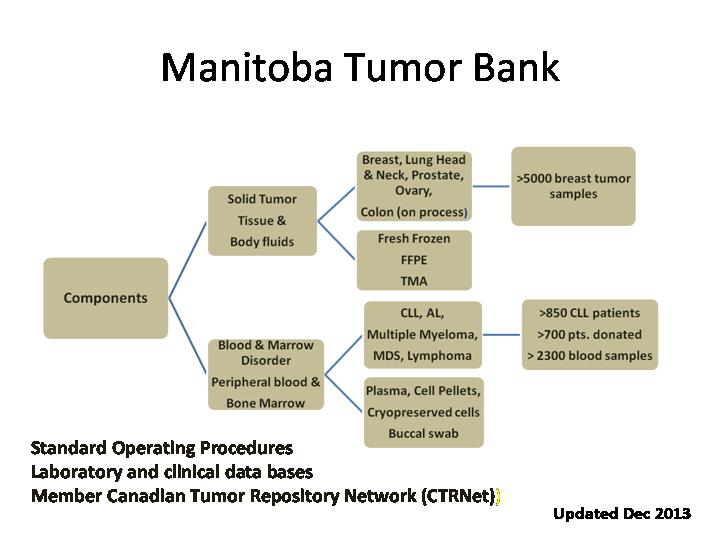
The Manitoba Tumour Bank is a collection of tissue and related clinical data. The Bank operates within the Department of Pathology of the Winnipeg Regional Health Authority and University of Manitoba, and CancerCare Manitoba. The Bank was originally established by the National Cancer Institute of Canada in 1993 with funds from the Canadian Cancer Society and is now supported by CancerCare Manitoba Foundation in partnership with the University of Manitoba and the Canadian Tissue Repository Network (CTRNet). The Bank provides an important resource both for breast cancer research at the University of Manitoba and for researchers across Canada and internationally.
What does the Manitoba Tumour Bank do?
During the assessment of each biopsy specimen small tissue samples are taken by Pathologists to process and examine under a microscope and these samples are then stored as a clinical archive.
After all diagnosis has been completed, the Bank organizes these tissues and related clinical data into 'cases' for both future research and future clinical purposes and stores these 'cases' in CancerCare Manitoba. Researchers can apply to study these cases only through a review process and if they obtain approval for their research project from an institutional ethics review board. If approved, researchers are provided with tissue sections and the related clinical information from a set of typically 100 or more 'cases'.
These cases are carefully selected from the computer database on the basis of selection criteria such as size and type of tumour that are relevant to the research question under study.
All cases are distinguished by a Tumour Bank number but are anonymous due to the absence of any tag that might allow it to be traced to an individual patient. Researchers are charged to cover the costs of storage and release but no tissue or information is sold.
The Bank has supported over 50 research studies on cancer across North America and Europe.
What information is stored and released?
The Bank stores three types of information on each case within a secure location in CancerCare Manitoba. This information relates to the tissue, clinical, and follow-up information. Tissue information includes the composition of the tissue, the size and type of tumour. Clinical information includes the patient age, clinical symptoms and the results of clinical tests such as x-rays. Follow-up information includes the type of treatment after surgery and the response to this treatment. Information is never released from the Bank with any label that might allow it to be traced to an individual. Information is only released as part of a set of anonymized cases, where each case is labelled by an anonymous tumour bank number and consists of a section of tissue with related information.
Accessing the Tumour Bank
Projects that use the resource fall into two general categories: i) Projects initiated by members of the Manitoba Breast Cancer Research Group and other academic researchers in Manitoba, ii) Projects initiated by external academic users in Canada, in the USA, and in Europe. Local academic users receive first priority. However, the MBTB design and philosophy has ensured that for over more than a decade of operation, external users have almost equivalent access.
Update
With the renewal of the Manitoba Breast Tumour Bank by CIHR in 2006, came an expanded mandate to collect from other disease sites. To date, the bank has accrued 55 Head and Neck cases, 80 lung cases with matched paraffin and frozen blocks. The Tumour Bank also developed standard operating protocols for collection and banking of prostate cancers and normal breast tissues from reduction mammaplasties. This collection began December 2009. To date, the bank has accrued 60 prostate cases and 30 normal breast tissue cases.
***Reminder to insert image***
MBTB Application Forms
Links to Other Canadian Tumour Banks
Operational Procedures
Collections
Cases are collected represent approximately 33% of 750 breast cancer cases per annum that are diagnosed and treated in Manitoba. Cases accrued are those that meet the following criteria; a) treated by surgical resection, b) tissue is submitted for pathological examination by Department of Pathology, DSM Laboratory Medicine Program c) excess tissue exists that would otherwise be discarded after completion and sign out of case, i.e. tissue is not required for clinical assessment/diagnosis.
This latter criteria is determined solely by the pathologist responsible for the case, not by the MTB. Once it has been determined that there is excess tissue available then this is accrued to the MTB. The tissue is then processed and assessed and the accompanying clinical data is also assessed to construct an MTB case. The clinical database is integrated with the provincial Cancer Registry.
Different case categories are crafted to allow the MTB to offer suitable cases with the appropriate extent of data for different types of research question. These categories are labelled A to D and are described in detail in tabular form in the application form. "A" cases are associated with an established protocol (collection on ice, documentation of time from end of surgery to freezing of tissue) and multiple tissue samples (eg from normal breast from the surgical specimen, primary tumour, and axillary lymph nodes). "B, C, D" category cases are mostly primary tumour samples and differ only in the extent of clinical data collected or extent of tissue available. All categories are associated with complete pathology, baseline clinical data. "A" and "B" subsets are also associated with extended treatment and outcome data. "D" cases derive from very large tumours with additional tissue blocks available for larger scale assays.
In all cases, tissue samples are obtained frozen and processed by the bank to produce several matching 'mirror image' paraffin and frozen tissue blocks. Further processing to create paraffin block 'Tissue Micro Arrays' is in progress from a subset of the paraffin block material.
***Reminder to Insert Images***
Location
The MTB is located at CancerCare Manitoba within dedicated space. This comprises a purpose designed laboratory area including a freezer room, darkroom, and office space.
There are two databases, one for tissue and clinical data associated with tissues stored in the MTB (the Manitoba Breast Cancer Database), and a second database for all breast surgical events in the province that are associated tissues in the clinical pathology archives (the Manitoba Breast Event Database). These are accessed through secured and password protected and firewall protected microcomputers.
Quality control
The MTB is evaluated through a regular process of internal evaluation to maintain the quality of this program. At the operations level this consists of quarterly Major Users Committee meetings in order to review all operational aspects. At a practical level we conduct regular audits on the integrity of the tissue storage and clinical database, and we have developed protocols for the quality control and efficient use of the tissues. A histological quality assessment is performed on all tissues and documented on all cases. An additional material quality assessment is performed on all cases using a semi-quantitative scoring system based on tumour cell nuclear parameters that relates to RNA quality. Our protocols for DNA, RNA & protein extraction from thin tissue sections are made readily available to investigators requesting material, in order to encourage the use of minimal amounts of tissue.
Confidentiality
An inherent property of any Tumour Bank is that it provides a recognized structure, open to external review and accountability, providing a secure, confidential insulation and interface between the patient and the researcher. The interface is governed by the Personal Health and Information Act of Manitoba and is managed by a qualified team.
The MTB database is maintained within the secure and confidential limits of CancerCare Manitoba. On the clinical interface it can thus be accessed within CancerCare Manitoba, where cases are potentially linkable to the patient to update the followup. This also allows for patient initiated withdrawal or patient or physician request for material for later clinical diagnostic purposes. On the research interface, cases distinguished only by an anonymous tumour bank number and chosen by anonymous selection criteria (eg type of tumour) are accessible only through a formal mechanism, scientific review, appropriate research ethics board approval.
A commitment is documented from each investigator and a representative of their institution that cases provided by the MTB will be used for research purposes only, that tissues and their products will not be sold or used for commercial purposes, or be distributed further to third parties for purposes of sale or producing for sale, cells or cell products. Furthermore, tissues or extracted material will not be used for any research project other than that described in the application.
Accessing the Tumour Bank
Projects that use the resource fall into two general categories: i) Projects initiated by members of the Manitoba Breast Cancer Research Group and other academic researchers in Manitoba, ii) Projects initiated by external academic users in Canada, in the USA, and in Europe. For both categories access to the MBTB involves three phases described below. Local academic users receive first priority. However, the MBTB design and philosophy has ensured that for over more than a decade of operation, external users have almost equivalent access.
Process
- Formal applications for access are required for all users. The application form includes:
- the investigator CV,
- sources of funding,
- description, design and justification of study,
- techniques to be used,
- the type and extent of data (histological, pathological and clinical) requested,
- evidence or plan for ethical review board approval,
- commitment to terms and conditions for use of material.
- Applications are reviewed by the MBTB Access Review Committee Committee that comprises the principal investigators of the University of Manitoba Breast Cancer Research Group and individuals with medical, and surgical oncology expertise. Review also encompasses the Pathology Access to Tissue Committee (PACT) on all applications that access paraffin blocks, and from the Cancer Care Manitoba Data Access committee on all applications that access clinical data. Criteria for review include the background and credentials of the investigator, evidence of peer reviewed and preferably national funding, high scientific quality and merit, and evidence of appropriate ethical review. The MBTB review panel considers ethical and confidentiality issues with respect to appropriate justification and equitable use of a rare resource donated by patients. However in accordance with TCPS and local REB policies, all applications are subject to documentation of appropriate and expert institutional REB approval. Projects are rated as supportable, supportable with reservations, or unsupportable.
- After review all applicants are notified of the panel decision and those that are supportable are asked to provide i) institutional and investigator signatures attesting to commitment to the terms of support, which include conditions on the secondary use of materials provided and recognition of MBTB support in publications arising from use of the material (see application form in appendix) and ii) documentation of appropriate institutional Research Ethics Board (REB) approval for the project.
- Prior to release of selected study materials, users are then sent a small test batch of tissues in the appropriate assay format (eg sections in tubes or on coated slides) to test courier mail 'connections' and the capability of the laboratory to successfully conduct their assays on MBTB material. Only after confirmation of successful receipt and performance of the relevant assay(s) on MBTB material is the study set finally sectioned and released in batches. Cases are identified only by an anonymous MBTB number. No clinical or demographic information (eg that concerns patient or family history or treatment center) that might compromise anonymity is released.
- Fees are assessed depending on the project.
Forms for Downloading
Fee Schedule
Currently under review
Research Units
Personnel
The overall operation of the MBTB is now directed by Dr. Leigh Murphy. The director and co-director have been assisted by the MTB coordinator, Michelle Parisien, and the following personnel:
- Pathologist - Dr. Carla Penner
- Lab Technician - Yun Li
- Lab Technician - Andrea Fristensky
- Lab Technician - Negin Hamadi
- Data Coordinator - Shannon Kornelsen
- Data Coordinator - Nicole Wozny
The Molecular Profiling Unit
The Molecular Profiling Unit contains technology platforms that are used to investigate gene expression at the RNA and protein levels, in multiple breast tumour biopsy samples using high through-put systems.
The unit contains several Ventana auto-staining machines for high through-put immunohistochemistry (IHC) and in situ hybridization (ISH) analyses of multi-tissue sections; an automated Tumour Imaging System which captures and documents high resolution images of the contents of the tumour sections that have been processed on the Ventana previously; a Nucleic Acid Workstation for the automated extraction of RNA and DNA from multiple samples; a DNA microchip reader which allows the measurement of expression of every gene in the human or mouse genome at the level of RNA in any tissue sample. So the capability of the unit is to profile multiple tumour samples either at the level of specific gene families through hypothesis driven research, or to more globally profile at the gene expression level to identify new patterns of gene expression which are associated with risk of disease, disease outcome and response to treatment. The unit has made further progress associated with molecular profiling of estrogen receptor isoforms in human breast cancer, identifying potentially better markers of responsiveness to endocrine therapies, identifying potential markers of risk of invasive breast cancer. The unit will cooperate with other platforms within the Breast Cancer Research Centre and MICB generally, and perform molecular profiling required by the research programs within and associated with the MICB.
Nygard International Molecular Biology Breast Cancer Research Unit
The proteomics facility in the Nygard International Breast Cancer Research Unit is equipped with Ciphergen Protein Chip mass spectrometer, HPLC, FPLC, real-time PCR, protein electrophoresis (1D and 2D) and Imager system to study the molecular biology of cancer, especially to identify biomarkers of breast cancer and prostate cancer. The Facility is involved in gene expression, protein purification and identification activities required in on-going cancer research projects. The Facility also services and helps the students and staffs in RIOH for their projects related to proteomics.
The Facility achieved to identify a protein, PRDX 1, that preferentially cross-linked to DNA in estrogen receptor negative (ER-) but not ER+ or normal breast epithelial cells. One paper (Selective Association of Peroxiredoxin 1 with Genomic DNA and COX-2 Upstream Promoter Elements in Estrogen Receptor Negative Breast Cancer Cells) was published on MBC in 2010. We also identified several DNA-bound proteins present only in metastatic prostate cancer cells. The Facility provided support to Dr. Leygue's, Dr. Murphy's, Dr. Mowat's, Dr. Gibson's, Dr. Mai's, Dr Hick's and Dr Raouf's students, postdoctoral fellows and technicians to perform real-time PCR, 2D protein electrophoresis, Imager analysis, and proteomics analysis.

